THE HEXAGONAL CELL STRUCTURE OF ICE-I
By Philip H.Starmer*
Charlotte, NC
e-mail: st9m6@windstream.net
ABSTRACT
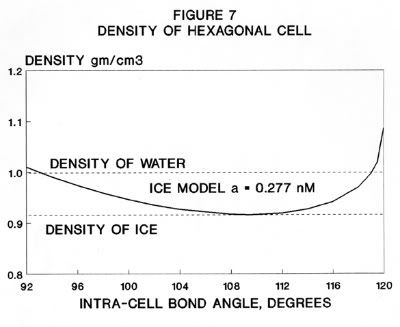
The structure of Ice-I consists of a layer of puckered hexagonal rings of water molecules that have the conformation of the chair form of cyclohexane. Using simple mathematics, an equation is derived which relates the volume of a hexagonal cell, containing six H2O molecules, with the distance between the nearest neighbor oxygen atoms. Differentiation of this equation leads to the fact that maximum volume of the cell, and thus the minimum density, occurs when the O - O - O angle equals the tetrahedral angle. The tetrahedral angle can be envisaged using an oxygen atom at the center of a cube with other atoms at opposite corners. The sine of half the tetrahedral angle is √(2/3) which gives avalue for the full angle of 109° 28' . Substituting the tetrahedral angle in the equation gives a volume of the cell as (16 a^3)/ √ 3 where a = the nearest neighbor O - O distance. Based on X-ray spectrography, the nearest neighbor O - O distance is 0.277 nanometers . Plugging this figure into the above equation gives a cell volume of 196.3*10^-24 cubic centimeters. The mass of six H2O molecules, taking into account of various isotopes, is 179.724 * 10 ^ -24 grams. And this gives a density of 0.915 gm/cc, which is close to the experimental value is 0.916 gm/cc. In a previous paper it was proposed that half the hydrogen bonds in ice are weak and half are strong with the weaker ones breaking when ice melts and the stronger ones breaking when water boils. This leads to the conclusion that liquid water is a cyclic structure of six H2O molecules. Further when ice melts the density increase can only be explained if the O - O - O angle increases from the tetrahedral angle to almost 120 degrees, implying an almost flat structure. It is postulated that at the temperature of maximum density the angle is 120 degrees and the structure is flat. And this is the point when the structure changes from a chair structure at lower temperatures to a boat structure at higher temperatures.
This model also gives a calculatedvalue for the residual entropy of Ice-I as 0.823 e.u. which is closer to the experimental value of 0.82 e.u than thevalue of 0.805 e.u. calculated by Pauling. Since the basic structure of diamond is similar to that of Ice-I the same mathematics can be used to calculate its density. The data gives a calculatedvalue of 3.54 gm./cm3 which is close to the experimentalvalue for diamond of 3.51 gm./cm3.
DISCUSSION.
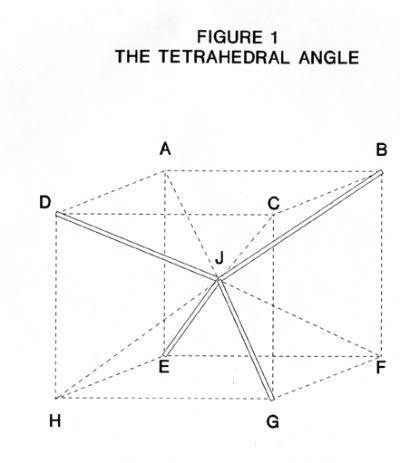
The angle between adjacent oxygen atoms in Ice-I is the tetrahedral angle(1) and this can demonstrated by the cube in Figure 1 (2). J, at the midpoint of the cube, represents an oxygen atom which is joined to its four nearest neighbors at points B, D, E and G such that J is equidistant from B, D, E and G. The angle between oxygen-oxygen-oxygen bonds, for example the angle BJD, is the tetrahedral angle. If the side of the cube is taken as unity then it can be calculated that the diagonal BD is √ 2 and BH is √ 3. Taking s as the tetrahedral angle, then
Sin (s/2) = 0.5BD/0.5BH (1)
= √ 2 / √ 3 (2)
= √(2/3) (3)
So s/2 = 54° 88' (4)
And s = 109° 28' (5)
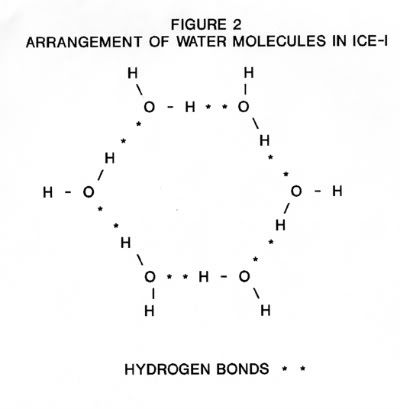
The structure of ice-I consists of layers of puckered hexagonal rings of oxygen atoms. Each oxygen atom is joined to two hydrogen atoms by covalent bonds and to two other hydrogen atoms by hydrogen bonds in a cyclic structure as shown in Figure 2. The six oxygen atoms are arranged in a puckered structure in the same manner as the chair structure of cyclohexane, as shown in Figure 3. A cell is defined as the structure enclosing each six-membered ring such that the boundaries of this cell are the mid-points of the oxygen-oxygen distances with neighboring cells and that there is no free space between the cells. The upper and lower surfaces of the cell are regular hexagons, as shown in Figure 4. Note, that the oxygen atoms are in two parallel planes (BDF and ACE in Figure 3) which are also parallel to the planes of the hexagon surfaces.
If the nearest oxygen-oxygen distance is a (e.g. AB in Figure 3) then the next nearest neighbor distance is b , (e.g. AC in Figure 3) then:
b = 2a.sin(T/2) (6)
Where T is the O - O - O bond angle between oxygen atoms within the cell (e.g. angle ABC in Figure 3).
All the bond angles within the cell are equal and this angle will be referred to as the intra-cell bond angle..
When T equals the tetrahedral angle we have from Equation (3) that sin(T/2) = √(2/3)
b = 2a.√(2/3) (7)
b = 1.633 a (8)
The second nearest neighbor is the distance of the diagonal c of the rectangle of sides of length a and b, for example, the rectangle BCEF in Figure 3. This distance is given by:
c = √(a² + b²) (9)
When T equals the tetrahedral angle we have, from Equation (7):
c =√(a² + 8a²/3) (10)
=√(11/3) . a (11)
= 1.915.a (12)
Narten et al. (3), using X-ray analysis, found a high concentrations of oxygen atoms at 0.277, 0.45 and 0.53 nanometers for Ice-I at 0° C. Taking thevalue of a = 0.277 nm so the next nearest neighbors calculate out to b = 0.451 nm., and c = 0.528 nm., which is in good agreement with the experimentalvalues.
The above information can now be used to calculate the volume of a hexagon cell containing six oxygen atoms arranged in a puckered ring with the associated hydrogen atoms.
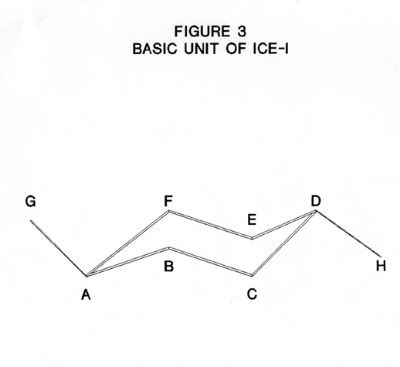
In Figure 3, s is the distance from a point half way between A and G ( in the adjacent cell on one side) to a point half way between D and H (in the adjacent cell on the other side). This distance is equal to DG and also to AH. It is twice the perpendicular of an equilateral triangle of sides b. So
s = 2 √ 3 b/2 (13)
s = √ 3 b (14)
s = 2 √ 3 sinT/2 a (15)
The volume of a hexagonal cell,V, is given by:
V = A * h (16)
where
A = the area of a regular hexagon and
.h = the thickness or height of the cell
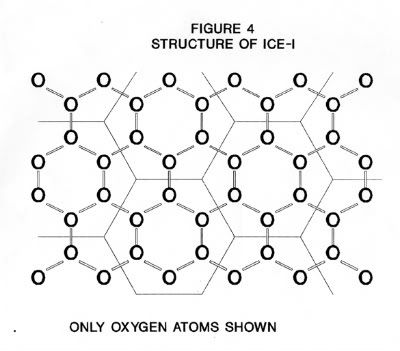
As seen from Figure 4, s is the distance between the sides of a regular hexagon, such as the sides AC, BD and CE is Figure 5. In the right-angle triangle ABD the side AB = 1/2 the diagonal AC. If the diagonal equals d then;
d² = s² + d²/4 (17)
d = 2s/ √ 3 (18)
= 4 (sin T/2) a (19)
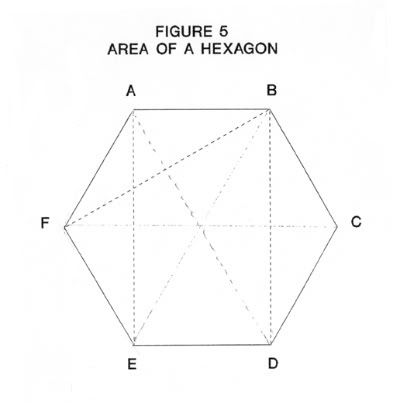
. In Figure 5, the area is made up of six equilateral triangles of side d/2 the area
A = 6 * 1/2 * d/2 * √ 3/4 *d (20)
A = 3 * √ 3/ 8 * d² (20A)
= 6* √ 3*sin²T/2 * a² (20B)
The height of the cell is
h = a/2 + p + a/2 (20C)
Where p is the distance between the plane of BDF and the plane of ACE in Figure 3. One a/2 is from half the distance from the plane of BDF to the unit above and the other is from half the distance from the plane of ACE to the unit below in Figure 3.
The distance p is the height of the pyramid CDEH which has a base an equlilateral triangle of sides b and has faces that are isosceles triangle of base b and sides a. In Figure 6a:
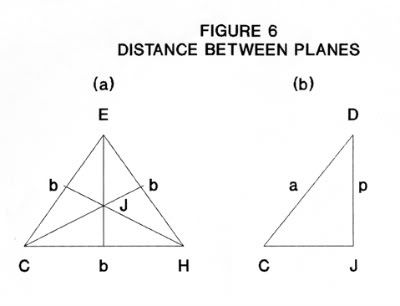
CJ = b/(2 cos30°) (21)
= b/ √ 3 (22)
Substituting the value of b from Equation (6)
CJ = 2a sin(T/2)/ √ 3 (23)
From Figure 6b
p = √( a^2 -CJ^2) (24)
p = a* √(1 - 4/3 * sin²T/2) (25)
Note, that when T = the tetrahedral angle p = a/3. Also when T = 120°, p = 0 so for this angle the six oxygen atoms are all in the same plane.
h = a + a* √(1 - 4/3 * sin²T/2) (26)
The volume of the hexagonal cell is given by the equation:
V = {1 + √(1 - 4/3 * sin²T/2)}{ 6*√ 3*sin²T/2 *}*a^3 (27)
Let x = sin T/2 to simplify the notation for differentiation
V = {1 + √(1 - 4/3 * x²)}{6*√ 3*x²}*a^3 (28)
dV/dx = {[1 + √(1 - 4/3 * x²)](12*√ 3 x) + (6*√ 3*x²)[0 + 1/2 (-8/3 x)/v(1-4/3x²)]}*a^3 (29)
When T equals the tetrahedral angle, x = √(2/3) Substituting this value in Equation (29) reduces to :
{ 16 √ 2 - 16 √ 2} a^3 (30)
Which is zero. In other words, the volume of the cell is maximum when the intra-cell angle is the tetrahedral angle.
When T equals the tetrahedral angle, Equation (27) reduces to:
V =16( a^3)/ √ 3 (31)
As noted above, the adjacent oxygen-oxygen distance, a, is 0.277 nm. Substituting this value in Equation (31) gives a volume of the cell as 0.196335 cubic nanometers or 196.335 * 10^-24 cubic centimeters. The mass of the H atom is 1.69346 * 10^-24 grams and the mass of the oxygen atom is 26. 5593 *10^-24 grams (4) so the mass of the hexagonal cell, taking into account small amounts of isotopes, is 179.724 *10^-24 grams. Dividing the mass by the volume gives a theoretical density of 0.915 gram/cubic centimeter which is close to the experimentalvalue is 0.916 gm/cc..
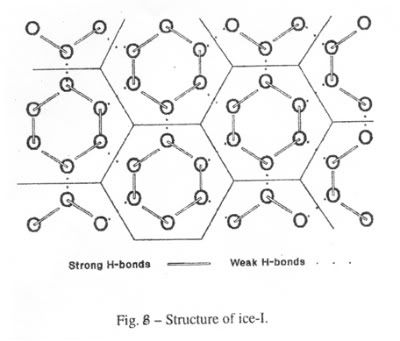
To explain the structure of liquid water, Starmer (5) proposed that the hydrogen bonds in Ice-I are unequal, as shown in Figure 8. The weak hydrogen bonds break when ice melts and the stronger ones persisting in water and break when water boils. In other words, the six membered hexagonal cell is the structure of liquid water. This leads to a model for liquid water as a cyclic structure of six H2O molecules with six oxygen atoms in a puckered ring joined by the strong hydrogen bonds. Such a model explains the apparent thermoplasticity of ice in glaciers with the breaking and subsequent recombining of the weak hydrogen bonds.
When Ice-I melts to water at zero degrees Celsius the O - O distance increases to 0.282 nm and the density increases to 0.999 gm/cc. If the O-O-O angle stayed at the tetrahedral angle the calculated volume of the cell would be 207.16 *10 ^-24 ccs..giving a density of 0.868 gm/cc. However, the density of 0.999 gm/cc indicates that the volume of the cell has shrunk to 179.9*10^-24 ccs.and this can only be obtained if the O-O-O angle is close to120°. As noted above, this implies that all the oxygen atoms are in the same plane or close to it. It was postulated (5), that the cell is, in effect, the structure of liquid water due to the breaking of the weak inter-cell hydrogen bonds. Since the weak hydrogen bonds have been inactivated the cells can move relative to one another in the same way as molecules of benzene or cyclohexane. Thus, liquid water has the structure (H2O)6. The various isotopic forms of water, H2O, D2O, T2O etc., are unique (1) in that for each there is a point of maximum density above and close to the freezing point. A possible explanation is that the point of maximum density is the point where the O-O-O angle equals 120° and the structure of the cell changes from the chair form in ice and water at lower temperatures to the boat form in liquid water at higher temperatures. As judged by viscosity, as shown in Table 1, water is closer to cyclohexane than it is to either benzene or normal hexane.
Giauque and Stout(6) determined the calorimetric and spectroscopicvalues of ice-I and found a difference of 0.82 e.u. (entropy units). Pauling(7) using a model with random orientation of hydrogen bonds, calculated the residual entropy of ice as 0.805 e.u. The above model offers a different approach.
There are N molecules of H2O in a mole of ice so there are N/6 cells of (H2O)6, where
N = Avogadro s Number. Next, there are a number of ways a hexagonal cell can be included in the ice structure:
1) as the arrangement of hydrogen atoms involved in hydrogen bonds as in Figure 2 or its mirror image - total of two.
2) with the oxygen atoms arranged in a chair form in one way, as in Figure 3, or upside down - a total of two.
3) There are three ways in which one hexagon can be imposed on the top of another so that the hydrogen bonds line up - a total of three.
Thus, the total number of variations is twelve. Therefore, the total number of permitted configurations of the crystal, W, is
W = 12 ^(N/6) (32)
The residual entropy, S, is calculated from the equation:
S = k*(ln W) (33)
where k = the Boltzmann constant.
Combining Equations (31) and (32)
S = k*[ln 12 ^ (N/6)] (34)
= (kN/6)* ln(12) (35)
Since kN = R the gas constant
= 1.9872 calories /mole
S = (1.9872/6)*ln(12) e.u. (36)
= 0.823 e.u (37)
It should be noted, in passing, that while diamond has a somewhat different crystal structure from that of ice-I it is based on the same basic hexagonal cell. The distance between adjacent carbon atoms in diamond is (from reference #3) 0.154 nm so from Equation (31) the volume of the hexagonal cell is 0.03374 cubic nanometers or 33.74 *10^-24 ccs. The mass of six carbon atoms is found to be 119.5 * 10^-24 gram. So the calculated density is 3.54 gram/cc. which is close to the experimentalvalue of 3.51 gram/cc.
REFERENCES
1) Eisenberg, D; Kauzmann,W., The Structure and Properties of Water, Oxford University Press, New York, ( 1969).
2) Daniels, F; Alberty, R.A., Physical Chemistry, 3rd Ed., Wiley., NewYork, ( 1966).
3) Narten, A.H.; Danford, M.D.; Levy , H.A., Discussions of the Faraday Soc., 43, (1967), 97 - 101.
4) Starling, S.G.; Woodall, A.J., Physics, Longmans, Green & Co., London (1950), p 1239.
5) Starmer, P.H., Indian J.Chem., 37A, November 1998, 1002 - 1005.
6) Giague, W.F.; Stout, J.W., J.Am.Chem.Soc., 58, (1936). 1144 - 1150.
7) Pauling, L., The Nature of the Chemical Bond, Cornell University Press, Ithaca, New York, 3rd Ed., (1960).
Table 1:
Viscosity - Centipoises
Temperature °C | Water | Cyclohexane | Benzene | Hexane |
15 | 1.1382 | 1.0730 | 0.7004 | 0.3243 |
25 | 0.8903 | 0.8980 | 0.6208 | 0.2923 |

No comments:
Post a Comment